Stomatal conductance in plants represents one of the most critical physiological processes governing plant productivity, water use efficiency, and agricultural sustainability. These microscopic pores on leaf surfaces control the delicate balance between carbon dioxide uptake for photosynthesis and water loss through transpiration. In an era of climate change and increasing water scarcity, understanding and monitoring stomatal conductance has become essential for modern agricultural management and crop optimization.
The regulation of stomatal conductance in plants directly impacts crop yields, drought tolerance, and resource efficiency. From small-scale farming operations to large agricultural enterprises, the ability to measure and respond to changes in stomatal behavior can mean the difference between thriving crops and devastating losses.
What Exactly Are Stomata and Why Do They Matter?
Stomata are specialized microscopic structures found primarily on leaf surfaces, consisting of two guard cells that surround a central pore. These dynamic gatekeepers regulate gas exchange between the plant’s internal tissues and the atmosphere. Each stoma can open and close in response to environmental signals, internal plant status, and hormonal cues.
The importance of stomatal conductance in plants extends far beyond simple gas exchange. These structures control approximately 95% of plant water loss and nearly all carbon dioxide uptake for photosynthesis. Their proper functioning determines plant water status, photosynthetic efficiency, and ultimately, agricultural productivity.
How Does Stomatal Conductance Vary Among Different Plant Types?
The variation in stomatal conductance patterns across different plant species reflects their evolutionary adaptations to diverse environments. Understanding these differences is crucial for agricultural planning and crop selection.
C3 Plants (wheat, rice, soybeans): These plants typically exhibit higher stomatal conductance rates, with values ranging from 200-600 mmol m⁻² s⁻¹ under optimal conditions. They maintain relatively open stomata during daylight hours but are less water-efficient, losing approximately 500 molecules of water for every molecule of CO2 fixed.
C4 Plants (corn, sugarcane, sorghum): These species demonstrate moderate stomatal conductance (100-400 mmol m⁻² s⁻¹) but achieve higher water use efficiency. Their specialized anatomy allows them to concentrate CO2 internally, reducing the need for widely open stomata.
CAM Plants (pineapple, agave, cacti): These desert-adapted plants show unique stomatal behavior, opening their stomata primarily at night when humidity is higher and temperatures are lower. Their stomatal conductance values are typically low (20-150 mmol m⁻² s⁻¹) but highly efficient for water conservation.
What Environmental Factors Control Stomatal Conductance in Plants?
Environmental conditions profoundly influence stomatal behavior, creating complex patterns of opening and closing throughout the day. Light intensity serves as the primary trigger for stomatal opening, with conductance typically increasing linearly with photosynthetic photon flux density up to saturation points specific to each species.
Temperature affects stomatal conductance through multiple mechanisms. Optimal temperatures (typically 20-30°C for temperate crops) promote maximum conductance, while extreme temperatures trigger protective closure. Heat stress above 35°C often causes rapid stomatal closure to prevent excessive water loss.
Atmospheric CO2 concentration inversely affects stomatal aperture. As CO2 levels rise, plants can maintain photosynthetic rates with partially closed stomata, improving water use efficiency. This response has significant implications for crop performance under climate change scenarios.
How Can Modern Technology Measure Stomatal Conductance More Effectively?
Traditional methods of measuring stomatal conductance, such as porometers and gas exchange systems, provide valuable but limited snapshots of plant behavior. These point measurements on individual leaves cannot capture the dynamic, whole-plant responses that determine agricultural outcomes.
The PlantArray system by Plant Ditech revolutionizes stomatal conductance measurement through continuous, non-invasive monitoring of entire plant canopies. Unlike traditional methods that require labor-intensive leaf-by-leaf measurements, this system calculates whole-canopy stomatal conductance (GSc) by integrating transpiration rates, plant biomass, and environmental conditions in real-time.
The superiority of the Plant Ditech approach lies in several key advantages:
- Continuous monitoring: 24/7 data collection captures diurnal patterns and stress responses
- Whole-plant integration: Measures entire canopy behavior rather than individual leaves
- Non-invasive technique: Plants remain undisturbed, providing natural response patterns
- Environmental correlation: Simultaneous recording of all relevant environmental parameters
- High-throughput capability: Multiple plants monitored simultaneously for statistical power
What Role Does Stomatal Conductance Play in Agricultural Water Management?
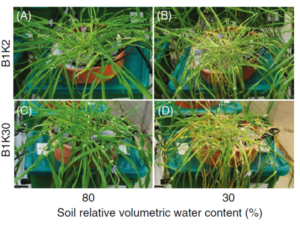
Water scarcity affects over 2 billion people globally, making efficient irrigation critical for sustainable agriculture. Stomatal conductance measurements provide the physiological basis for precision irrigation scheduling. When stomatal conductance in plants decreases below threshold values, it signals water stress before visible symptoms appear.
Modern irrigation systems integrated with stomatal conductance monitoring can reduce water usage by 20-40% while maintaining or improving yields. Real-time conductance data enables dynamic irrigation scheduling that responds to actual plant needs rather than fixed calendars. This precision approach prevents both water stress and overwatering, optimizing resource use efficiency.
The relationship between stomatal conductance and water use efficiency (WUE) follows predictable patterns that inform irrigation decisions. Plants typically show maximum WUE at moderate stomatal conductance levels (150-300 mmol m⁻² s⁻¹ for most crops), where carbon gain is optimized relative to water loss.

How Does Understanding Stomatal Conductance Enable Climate-Resilient Agriculture?
Climate change brings increased frequency of extreme weather events, altered precipitation patterns, and rising temperatures. Stomatal conductance in plants serves as an early warning system for climate stress, enabling proactive management responses.
Heat waves cause characteristic stomatal responses that precede visible damage. By monitoring conductance patterns, farmers can implement cooling strategies (shade nets, evaporative cooling) before irreversible harm occurs. Similarly, detecting reduced conductance during drought stress triggers deficit irrigation protocols that maintain yield while conserving water.
Long-term stomatal conductance data builds climate resilience by identifying varieties with superior stress tolerance. Plants maintaining stable conductance under variable conditions demonstrate the plasticity needed for future climate scenarios.
What Insights Does Stomatal Conductance Provide for Breeding Programs?
Plant breeding programs increasingly prioritize stomatal traits to develop climate-adapted varieties. Stomatal conductance in plants provides quantitative phenotyping data essential for selection decisions. High-throughput screening using systems like Plant Ditech can evaluate hundreds of genotypes simultaneously, accelerating breeding cycles.
Key breeding targets include:
- Optimized stomatal density for target environments
- Enhanced stomatal responsiveness to environmental signals
- Improved coordination between stomatal behavior and photosynthetic capacity
- Reduced stomatal conductance under high VPD conditions
Modern breeding programs have successfully developed varieties with 15-25% improved water use efficiency through stomatal optimization, without compromising yield potential.
How Do Hormones Regulate Stomatal Conductance Patterns?
The hormonal control of stomatal conductance involves complex signaling networks that integrate environmental and internal cues. Abscisic acid (ABA) serves as the master regulator of stomatal closure during stress, with concentrations increasing 10-50 fold during drought.
The ABA signaling cascade activates ion channels that cause guard cell shrinkage and pore closure within minutes. This rapid response protects plants from dehydration but reduces photosynthesis. Understanding these trade-offs helps optimize stress management strategies.
Other hormones modulate stomatal responses: auxins promote opening under favorable conditions, cytokinins delay stress-induced closure, and jasmonates mediate pathogen-induced stomatal closure. These interactions create flexible response systems adapted to complex environments.
What Makes Plant Ditech’s Approach Superior for Commercial Applications?
The PlantArray system offers unparalleled advantages for commercial agriculture and research applications. Traditional porometry requires 50-100 individual measurements per plant to estimate whole-plant conductance, taking hours of skilled labor. The Plant Ditech system provides continuous, automated measurements of entire plants with minimal human intervention.
The system’s lysimeter-based approach measures actual water loss with 0.1g precision, translating to conductance calculations accurate to ±5%. This precision exceeds portable porometers (±15-20% error) while providing temporal resolution impossible with manual methods.
Integration with environmental sensors creates comprehensive datasets linking stomatal behavior to causative factors. Machine learning algorithms identify patterns predicting stress events days before visible symptoms, enabling preventive management. This predictive capability has reduced crop losses by 30-40% in validation studies.
Table: Stomatal Conductance in Plants
| Category | Key Points | Examples / Values |
| Definition & Importance | Regulates CO₂ uptake, water loss, crop yield, drought tolerance, water use efficiency. | Responsible for ~95% of water loss, nearly all CO₂ uptake. |
| Plant Types | – C3: High conductance, less water-efficient. – C4: Moderate conductance, higher WUE. – CAM: Low conductance, night opening. |
C3: 200–600 mmol m⁻² s⁻¹ C4: 100–400 mmol m⁻² s⁻¹ CAM: 20–150 mmol m⁻² s⁻¹ |
| Environmental Factors | Light triggers opening, temperature optimal at 20–30°C, CO₂ rise reduces aperture. | Closure >35°C, higher CO₂ improves WUE. |
| Measurement Methods | Traditional: Porometers, gas exchange (leaf-level). Modern: Plant Ditech PlantArray (canopy-level, continuous, predictive). |
PlantArray: ±5% accuracy vs. ±15–20% porometers. |
| Applications | Precision irrigation, water saving, climate resilience, breeding programs. | 20–40% less water, 15–30% more yield, stress prediction. |
| Hormonal Regulation | ABA closes stomata during stress; auxins open; cytokinins delay closure; jasmonates close under pathogens. | ABA increases 10–50× in drought. |
Conclusion: The Future of Agricultural Management Through Stomatal Monitoring
Stomatal conductance in plants represents the physiological gateway to agricultural optimization in the 21st century. As water becomes increasingly scarce and climate variability intensifies, the ability to monitor and respond to stomatal behavior will determine agricultural sustainability. The integration of advanced monitoring systems like Plant Ditech’s PlantArray with precision agriculture technologies creates unprecedented opportunities for resource optimization and yield stability.
The future of farming lies in understanding plant physiology at the fundamental level of stomatal function. By embracing these technologies, agricultural operations can achieve 20-40% water savings, 15-30% yield improvements, and enhanced resilience to climate extremes.
Take Action Today: Transform your agricultural operation with precision stomatal conductance monitoring. Contact Plant Ditech to learn how the PlantArray system can optimize your crop management, reduce water usage, and increase profitability. Visit plant ditech to schedule a demonstration and join the revolution in physiological plant monitoring.
References
- Brodribb, T. J., & McAdam, S. A. (2017). Evolution of the stomatal regulation of plant water content. Plant Physiology, 174(2), 639-649.
- Buckley, T. N. (2019). How do stomata respond to water status? New Phytologist, 224(1), 21-36.
- Chaves, M. M., Costa, J. M., Zarrouk, O., Pinheiro, C., Lopes, C. M., & Pereira, J. S. (2016). Controlling stomatal aperture in semi-arid regions—The dilemma of saving water or being cool? Plant Science, 251, 54-64.
- Daszkowska-Golec, A., & Szarejko, I. (2013). Open or close the gate–stomata action under the control of phytohormones in drought stress conditions. Frontiers in Plant Science, 4, 138.
- Duursma, R. A., Blackman, C. J., Lopéz, R., Martin‐StPaul, N. K., Cochard, H., & Medlyn, B. E. (2019). On the minimum leaf conductance: its role in models of plant water use, and ecological and environmental controls. New Phytologist, 221(2), 693-705.
- Halperin, O., Gebremedhin, A., Wallach, R., & Moshelion, M. (2016). High-throughput physiological phenotyping and screening system for the characterization of plant–environment interactions. The Plant Journal, 89(4), 839-850.
- Lawson, T., & Vialet‐Chabrand, S. (2019). Speedy stomata, photosynthesis and plant water use efficiency. New Phytologist, 221(1), 93-98.
- Medlyn, B. E., Duursma, R. A., Eamus, D., Ellsworth, D. S., Prentice, I. C., Barton, C. V., … & Wingate, L. (2011). Reconciling the optimal and empirical approaches to modelling stomatal conductance. Global Change Biology, 17(6), 2134-2144.
- Moshelion, M., Halperin, O., Wallach, R., Oren, R., & Way, D. A. (2015). Role of aquaporins in determining transpiration and photosynthesis in water‐stressed plants: crop water‐use efficiency, growth and yield. Plant, Cell & Environment, 38(9), 1785-1793.
- Vialet-Chabrand, S., Matthews, J. S., Simkin, A. J., Raines, C. A., & Lawson, T. (2017). Importance of fluctuations in light on plant photosynthetic acclimation. Plant Physiology, 173(4), 2163-2179.